Electron injection dynamics in DSSCs
DSSCs are composed of a wide band gap semiconductor sensitized by a dye absorbing in the visible spectral region and have reached an efficiency exceeding 12%. The efficiency of a DSSC depends on a variety of kinetically competing charge-transfer mechanisms. The rate of each mechanism depends on the energy levels at the interfaces, the materials used, their morphology and preparation conditions as well as the chemical interactions between them.
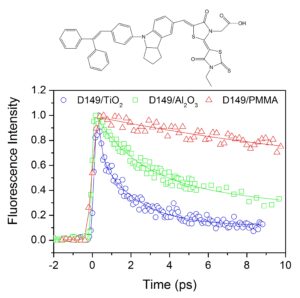 We are interested in studying the electron injection dynamics from the dye sensitizer to the oxide semiconductor substrate using femtosecond spectroscopy. We have investigated the injection dynamics from the very promising organic sensitizer D149 toward TiO2 nanocrystalline substrate as well as in various inert environments to understand the fundamental photodynamics of this dye.
We are interested in studying the electron injection dynamics from the dye sensitizer to the oxide semiconductor substrate using femtosecond spectroscopy. We have investigated the injection dynamics from the very promising organic sensitizer D149 toward TiO2 nanocrystalline substrate as well as in various inert environments to understand the fundamental photodynamics of this dye.
Our group has also studied the photodynamics of cation generation in dye sensitized TiO2 and 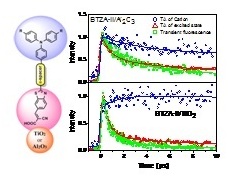 Al2O3 films by means of fs transient absorption spectroscopy in the visible with white-light probe. A new series of promissing organic sensitizers has been used bearing tri-phenylamine as electron donating grroup and benzothiazole as electron acceptor. We have found that cations are generated in both substrates meaning that electron injection is operative not only in TiO2 but also in Al2O3. The latter is used as a reference substrate and electrons are considered to be injected to trap states. Also, faster recombination of injected electrons takes place in Al2O3 dye-coated films as observed through fs transient absorption in the visible and IR.
Al2O3 films by means of fs transient absorption spectroscopy in the visible with white-light probe. A new series of promissing organic sensitizers has been used bearing tri-phenylamine as electron donating grroup and benzothiazole as electron acceptor. We have found that cations are generated in both substrates meaning that electron injection is operative not only in TiO2 but also in Al2O3. The latter is used as a reference substrate and electrons are considered to be injected to trap states. Also, faster recombination of injected electrons takes place in Al2O3 dye-coated films as observed through fs transient absorption in the visible and IR.
Another issue that should be taken into account is that the photophysics and solar cells efficiency of organic sensitizers comprising a cyanoacrylic acid group are greatly influenced by an equilibrium between the neutral (”non-deprotonated”, COOH) and anionic (“deprotonated”, COO–) forms, whose ratio depends on the solvent polarity and its H-bonding properties, dye concentration and temperature used.
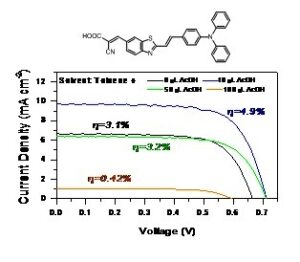 In a paper of ours published in JPCC, we report a detailed investigation on the relationship between the portions of COOH and COO– dye forms and the photophysical and solar cell properties of an organic dipolar sensitizer, BTZA-II, bearing triphenylamine electron-donating and benzothiazole electron-withdrawing moieties. The photophysics has been studied by stationary and time-resolved fluorescence spectroscopy in apolar and polar solvents with a dye concentration ranging from 5´10-7 M to 5´10-5 M, also upon addition of small amounts of an external acid or base in order to change the solvent acidity, allowing us to distinguish the contribution and lifetime of the neutral and anionic form. Also, solar cells using a solid-state electrolyte were prepared from toluene and toluene+acid solutions. A significant increase of the solar cell efficiency by 58% (η reaching a value of 4.9%) has been achieved after addition of an appropriate amount of acetic acid into the initial BTZA-II dye solution.
In a paper of ours published in JPCC, we report a detailed investigation on the relationship between the portions of COOH and COO– dye forms and the photophysical and solar cell properties of an organic dipolar sensitizer, BTZA-II, bearing triphenylamine electron-donating and benzothiazole electron-withdrawing moieties. The photophysics has been studied by stationary and time-resolved fluorescence spectroscopy in apolar and polar solvents with a dye concentration ranging from 5´10-7 M to 5´10-5 M, also upon addition of small amounts of an external acid or base in order to change the solvent acidity, allowing us to distinguish the contribution and lifetime of the neutral and anionic form. Also, solar cells using a solid-state electrolyte were prepared from toluene and toluene+acid solutions. A significant increase of the solar cell efficiency by 58% (η reaching a value of 4.9%) has been achieved after addition of an appropriate amount of acetic acid into the initial BTZA-II dye solution.
see:
“Femtosecond Decay and Electron Transfer Dynamics of the Organic Sensitizer D149 and Photovoltaic Performance in Quasi-Solid State Dye-Sensitized Solar Cells” M. Fakis, E. Stathatos, G. Tsigaridas, V. Giannetas and P. Persephonis Journal of Physical Chemistry C 115 (2011) 13429-13437.
“Interfacial electron transfer dynamics and photovoltaic performance of TiO2 and ZnO solar cells sensitized with Coumarin 343” M. Giannouli and M. Fakis, Journal of Photochemistry and Photobiology A: Chemistry 226 (2011) 42-50.
“A time resolved fluorescence and quantum chemical study of the solar cell sensitizer D149” M. Fakis, P. Hrobárik, E. Stathatos, V. Giannetas and P. Persephonis Dyes and Pigments 96 (2013) 304-312.
“Electron injection in TiO2 films and quasi-solid state solar cells sensitized with a dipolar fluorene organic dye” M. Fakis, M. Dori, E. Stathatos, Hsien-Hsin Chou, Yung-Sheng Yen, Jiann T’suen Lin, V. Giannetas and P. Persephonis Journal of Photochemistry and Photobiology A: Chemistry 251 (2013)18–24.
“Electron injection studies on TiO2 nanocrystalline films sensitized with fluorene dyes and photovoltaic characterization. The effect of co-adsorption of a bile acid derivative. ” M. Dori, K. Seintis, E. Stathatos, G. Tsigaridas, T.-Y. Lin, J. T. Lin, M. Fakis, V. Giannetas and P. Persephonis Chemical Physics Letters 563 (2013) 63–69.
“Synthesis of two tri-arylamine derivatives as sensitizers in dye-sensitized solar cells: Electron injection studies and photovoltaic characterization” M. Can, Z. Yigit, D. Karageorgopoulos, K. Seintis,, V. Giannetas, S. Demic, M. Fakis and E. Stathatos Synthetic Metals 188 (2014) 77– 85
“Excited state and injection dynamics of tri-phenylamine sensitizers with benzothiazole electron accepting group. A transient absorption and time resolved fluorescene study” M. Fakis, P. Hrobarik, I. Sigmulova, E. Stathatos and E. Vauthey JPCC 118 (2014) 28509–28519
“The effect of additional electron donating group on the photophysics and photovoltaic performance of two new metal free D-<pi>-A sensitizers” An. Margalias, K. Seintis, M.Z. Yigit, M. Can, D. Sygkridou, V. Giannetas, M. Fakis, E. Stathatos Dyes and Pigments 121 (2015) 316-327
“Solvent-Acidity-Driven Change in Photophysics and Significant Efficiency Improvement in Dye-Sensitized Solar Cells of a Benzothiazole-Derived Organic Sensitizer” by K. Seintis, Ç. Şahin, I. Sigmundová, E. Stathatos, P. Hrobárik, M. Fakis J. Physical Chemistry C 122 (2018) 20122−20134.
Ultrafast dynamics in Organic Solar Cells
Organic solar cells are becoming very attractive for cost-effective alternatives to Si-based photovoltaics as they offer potential for large-scale and inexpenssive manufacturing on flexible plastic substrates. Bulk heterojunction organic photovoltaics in particular are the subject of an extensive research effort. Towards improving their efficiency anode and cathode interlayers are being used for efficient transport of charge carriers after the dissociation of the electron-hole pair (exciton).
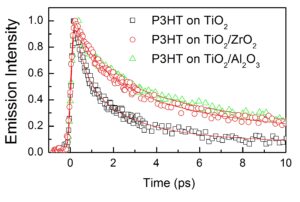 We are interested in studying the ultrafast dynamics of P3HT and of the dissociation of excitons in bulk heterojunctions composed of P3HT-PCBM with various interlayers. In a recent paper published in Adv. En. Mater. Al2O3 and ZrO2 ALD metal oxide substrates have been used to pasivate surface trap states of TiO2 films used as cathode interlayers. A clear increase in exciton lifetime is observed when P3HT and blend films are deposited on TiO2/ALD substrates which means that the effective photocarrier lifetimes are also increased.
We are interested in studying the ultrafast dynamics of P3HT and of the dissociation of excitons in bulk heterojunctions composed of P3HT-PCBM with various interlayers. In a recent paper published in Adv. En. Mater. Al2O3 and ZrO2 ALD metal oxide substrates have been used to pasivate surface trap states of TiO2 films used as cathode interlayers. A clear increase in exciton lifetime is observed when P3HT and blend films are deposited on TiO2/ALD substrates which means that the effective photocarrier lifetimes are also increased.
In a paper published in Org. Electronics we investigate the optoelectronic properties of a donor-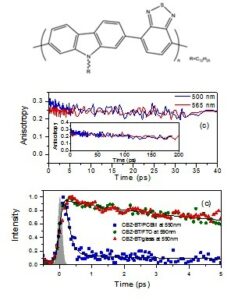 acceptor poly(N-Dodecyl-2,7-carbazole-alt-benzothiadiazole (CBZ-BT) copolymer in solutions and thin films. Absorption spectroscopy revealed two bands at 322/338 and 445/475 nm for CBZ-BT in solutions/films attributed to the carbazole and benzothiadiazole groups respectively. Time resolved spectroscopy in solutions reveals a transient red-shift of the emission spectrum within less than 5 ps due to exciton migration and/or conformational relaxation of the polymer backbone. In films, this relaxation is faster accompanied by a quenching of the exciton lifetime. Fluorescence depolarization in solutions follows the rate of spectral relaxation. In films, the overall depolarization is faster leading to a reduced limiting anisotropy, due to efficient energy transfer to adjacent chains with different polarization of the transition dipoles and increased disorder in the solid state. An almost complete quenching of the copolymer fluorescence, taking place on ~ 150 fs, was observed upon blending with a fullerene PC70BM acceptor pointing to an efficient electron transfer.
acceptor poly(N-Dodecyl-2,7-carbazole-alt-benzothiadiazole (CBZ-BT) copolymer in solutions and thin films. Absorption spectroscopy revealed two bands at 322/338 and 445/475 nm for CBZ-BT in solutions/films attributed to the carbazole and benzothiadiazole groups respectively. Time resolved spectroscopy in solutions reveals a transient red-shift of the emission spectrum within less than 5 ps due to exciton migration and/or conformational relaxation of the polymer backbone. In films, this relaxation is faster accompanied by a quenching of the exciton lifetime. Fluorescence depolarization in solutions follows the rate of spectral relaxation. In films, the overall depolarization is faster leading to a reduced limiting anisotropy, due to efficient energy transfer to adjacent chains with different polarization of the transition dipoles and increased disorder in the solid state. An almost complete quenching of the copolymer fluorescence, taking place on ~ 150 fs, was observed upon blending with a fullerene PC70BM acceptor pointing to an efficient electron transfer.
see:
“Solution processed hydrogen molybdenum bronzes as highly conductive anode interlayers in efficient organic photovoltaics” A. Soultati, A. M. Douvas, D. G. Georgiadou, L. C. Palilis, J. M. Feckl, S. Gardelis, M. Fakis, S. Kennou, P. Falaras, T. Stergiopoulos, N. A. Stathopoulos, D. Davazoglou, P. Argitis and M. Vasilopoulou Advanced Energy Materials 4 (2014) 1300896
“Porphyrin oriented self-assembled nanostructures for efficient exciton dissociation in highly performing organic photovoltaics” M. Vasilopoulou, D. G. Georgiadou, A. M. Douvas, A. Soultati, V. Constantoudis, D. Davazoglou, S. Gardelis, L. C. Palilis, M. Fakis, S. Kennou, T. Lazarides, A. G. Coutsolelos and P. Argitis J. Mater. Chem. A 2 (2014) 182–192
“Atomic Layer Deposited Aluminum and Zirconium Oxides for Surface Passivation of TiO2 in High-Efficiency Organic Photovoltaics” M. Vasilopoulou, D. G. Georgiadou, A. Soultati, N. Boukos, S. Gardelis, L. C. Palilis, M. Fakis, G. Skoulatakis, S. Kennou, M. Botzakaki, S. Georga, C. A. Krontiras, F. Auras, D. Fattakhova-Rohlfing, T. Bein, T. A. Papadopoulos, D. Davazoglou, P. Argitis Advanced Energy Materials (2014)1400214
“Surface Modification of ZnO Layers via Hydrogen Plasma Treatment for Efficient Inverted Polymer Solar Cells” V. Papamakarios, E. Polydorou, A. Soultati, N. Droseros, D. Tsikritzis, A. Douvas, L. Palilis, M. Fakis, S. Kennou, P. Argitis and M. Vasilopoulou ACS Applied Materials and Interfaces 8 (2016) 1194–1205
“Plasma induced degradation and surface electronic structure modification of Poly(3-hexylthiophene) films” M. Tountas, D. G. Georgiadou, A. Zeniou, K. Seintis, A. Soultati,E. Polydorou, S. Gardelis, A. M. Douvas, T. Speliotis, D. Tsikritzis, S. Kennou, M. Fakis, E. Gogolides, D. Tsoukalas, P. Argitis, M. Vasilopoulou Polymer Degradation and Stability Polymer Degradation and Stability 149 (2018) 162-172
“Photophysics, Electronic Structure and Solar Cell Performance of a Donor-Acceptor Poly(N-dodecyl-2,7-carbazole-alt-benzothiadiazole)” A. Koutsoubelitis, K. Seintis, D. Tsikritzis, J. Oriou, C. Brochon, E. Cloutet, G. Hadziioannou, M. Vasilopoulou, S. Kennou, M. Fakis, L. C. Palilis Organic Electronics 59 (2018) 202-212
“Lithium Doping of ZnO for High Efficiency and Stability Fullerene and Non-fullerene Organic Solar Cells” A. Soultati, A Fakharuddin, E. Polydorou, C. Drivas, A. Kaltzoglou, M. Irfan Haider, F. Kournoutas, M. Fakis, L. C. Palilis, S. Kennou, D. Davazoglou, P. Falaras, P. Argitis, S. Gardelis, A. Kordatos, A. Chroneos, L. Schmidt-Mende, M. Vasilopoulou, ACS Appl. Energy Mater. 2 (2019) 1663-1675
“Organic solar cells of enhanced efficiency and stability using zinc oxide:zinc tungstate nanocomposite as electron extraction layer” A. Soultati, A. Verykios, T. Speliotis, M. Fakis, I. Sakellis, H. Jaouani, D. Davazoglou, P. Argitis, M. Vasilopoulou, Organic Electronics 71 (2019) 227–237
Photophysics of self-assembled multichromophoric systems
The realization of supramolecular multi-chromophoric structures with fixed separation and orientation between the chromophoric building blocks is the structural basis for maximizing chromophore communication – through donor/acceptor electronic energy transfer phenomena. – while thwarting concentration quenching. These structural features would allow the direction of the electronic energy flow between distinct spatially organizing pairs to be dictated by tailoring the electronic transition-energy gaps of the donor/acceptor chromophoric building blocks.
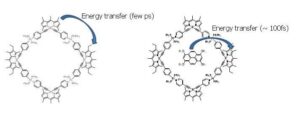 In this topic, we are interested in studying the photophysics and photodynamics in such systems with fs spectroscopy in order to determine straightforwardly the excitation energy transfer rates involved in the above supramolecular multichromophoric arrays. For example, we have studied the rhomboidal structure shown which is composed by a pair of tectons – the Bodipy 1 (donor) and the Bodipy 2 (acceptor). We have also studied the guest-host system in
In this topic, we are interested in studying the photophysics and photodynamics in such systems with fs spectroscopy in order to determine straightforwardly the excitation energy transfer rates involved in the above supramolecular multichromophoric arrays. For example, we have studied the rhomboidal structure shown which is composed by a pair of tectons – the Bodipy 1 (donor) and the Bodipy 2 (acceptor). We have also studied the guest-host system in 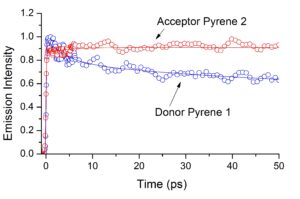 which a tetrasulfonated derivative of pyrene has been incorporated within the cavity of the rhomboidal structure. We have found that energy transfer between the bodipy tectons takes place within few ps. Additionally, the pyrene derivative directly transfers energy to Bodipy 2 within a few 100 fs.
which a tetrasulfonated derivative of pyrene has been incorporated within the cavity of the rhomboidal structure. We have found that energy transfer between the bodipy tectons takes place within few ps. Additionally, the pyrene derivative directly transfers energy to Bodipy 2 within a few 100 fs.
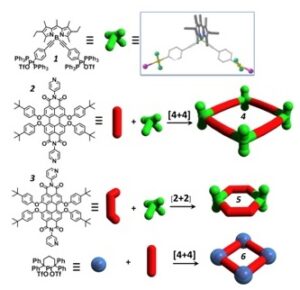 In a recent paper we have studied the photophysics of self-assembled polygonal hosts where bodipy chromophores are placed at the corners and are aligned perpendicular to the plane of the polygonal formed by perylene bisimide chromophores. Energy transfer
In a recent paper we have studied the photophysics of self-assembled polygonal hosts where bodipy chromophores are placed at the corners and are aligned perpendicular to the plane of the polygonal formed by perylene bisimide chromophores. Energy transfer 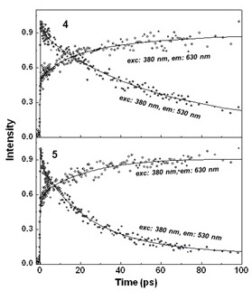 phenomena bethween the orthogonally placed chromophores were investigated by fs time resolved fluorescence spectroscopy accompagnied by anisotropy dynamics. Efficient energy transfer from the donor bodipy to the acceptor perylene bisimide was observed in 4 and 5 with lifetimes of the order of 55 and 30 ps respectively. Time-resolved fluorescence anisotropy studies, moreover, demonstrate fast excitation energy hopping, leading to a rapid excited state equilibrium among the low energy perylene-bisimide (PBI) chromophores.
phenomena bethween the orthogonally placed chromophores were investigated by fs time resolved fluorescence spectroscopy accompagnied by anisotropy dynamics. Efficient energy transfer from the donor bodipy to the acceptor perylene bisimide was observed in 4 and 5 with lifetimes of the order of 55 and 30 ps respectively. Time-resolved fluorescence anisotropy studies, moreover, demonstrate fast excitation energy hopping, leading to a rapid excited state equilibrium among the low energy perylene-bisimide (PBI) chromophores.
Multichromophoric systems with energy donor-acceptor chromophores attract tremendous scientific and technological interest as prototype materials towards understanding how energy is captured and selectively transferred to specific centees. Excitation energy transfer (EET) plays a crucial role in such systems as it is the fundamental phenomenon by which the energy harvested by molecular antennae systems is funneled to lower energy acceptors where secondary photochemical/photophysical phenomena take place.
 In collaboration with the group of Eric Vauthey, we have studied the photophysical properties of two pyrene-bodipy molecular dyads, composed of a phenyl-pyrene (Py-Ph) linked to the meso
In collaboration with the group of Eric Vauthey, we have studied the photophysical properties of two pyrene-bodipy molecular dyads, composed of a phenyl-pyrene (Py-Ph) linked to the meso
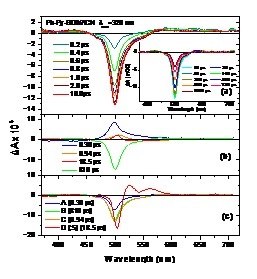
position of a bodipy (BD) molecule with either H-atoms (BD1) or ethyl groups (BD2) at the 2, 6 positions, by stationary, nanosecond and femtosecond spectroscopy. Excitation of the Py subunit eads to emission that is totally governed by the BD subunits in both dyads and in both dichloroethane and acetonitrile, pointing to excitation energy transfer (EET) from the Py to BD chromophore. Femtosecond fluorescence and transient absorption spectroscopy 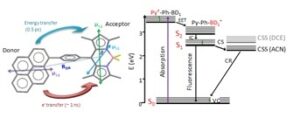 reveal that EET takes place within 0.3-0.5 ps and is mostly independent of the solvent and the type of the BD subunit. The EET lifetime is in reasonable agreement with that predicted by Förster theory. After EET has taken place, Py-Ph-BD1 in DCE and Py-Ph-BD2 in both solvents decay mainly radiatively to the ground state with 3.5 – 5.0 ns lifetimes which are similar to those of the individual BD chromophores. However, the excited state of Py-Ph-BD1 in ACN is quenched having a lifetime of 1 ns. This points to the opening of an additional non-radiative channel of the excited state of Py-Ph-BD1 in this solvent, most probably charge separation (CS). Target analysis of the TA spectra has shown that the CS follows an inverted kinetics and is substantially slower than the recombination of the charge-separated state.
reveal that EET takes place within 0.3-0.5 ps and is mostly independent of the solvent and the type of the BD subunit. The EET lifetime is in reasonable agreement with that predicted by Förster theory. After EET has taken place, Py-Ph-BD1 in DCE and Py-Ph-BD2 in both solvents decay mainly radiatively to the ground state with 3.5 – 5.0 ns lifetimes which are similar to those of the individual BD chromophores. However, the excited state of Py-Ph-BD1 in ACN is quenched having a lifetime of 1 ns. This points to the opening of an additional non-radiative channel of the excited state of Py-Ph-BD1 in this solvent, most probably charge separation (CS). Target analysis of the TA spectra has shown that the CS follows an inverted kinetics and is substantially slower than the recombination of the charge-separated state.
see:
“Highly efficient and unidirectional energy transfer within a tightly self-assembled host-guest multichromophoric array” N. Karakostas, I. M. Mavridis, K. Seintis, M. Fakis, E. N. Koini, I. D. Petsalakis and G. Pistolis Chem. Commun. 50 (2014) 1362-1365
“Energy Transfer within Self-Assembled Cyclic Multichromophoric Arrays Based on Orthogonally Arranged Donor – Acceptor Building Blocks” N. Karakostas, E. Martinou, A. Kaloudi Chantzea, K. Seintis, H. Oberacher, F. Pitterl Florian, M. Fakis, J. K. Kallitsis and G. Pistolis Faraday Discussions 185 (2015) 433-454
“Formation of a A Highly–Ordered Rigid Multichromophoric 3D Supramolecular Network by Combining Ionic and Coordination – Driven Self – Assembly” by A. Kaloudi-Chantzea, E. Martinou, K. Seintis, N. Karakostas, P. Giastas, F. Pitterl, H. Oberacher, M. Fakis and G. Pistolis Chemical Communications 52 (2016) 3388-3391
“The Dynamics of Intramolecular Energy Hopping in Multi-Bodipy Self – Assembled Metallocyclic Species: A Tool For Probing Subtle Structural Distortions in Solution” E. Martinou, K. Seintis, N. Karakostas, A. Bletsou, N. Thomaidis, M. Fakis and G. Pistolis Journal of Physical Chemistry C 121 (2017) 5341–5355
“Energy Transfer and Charge Separation Dynamics in Photoexcited Pyrene-Bodipy Molecular Dyads” M. Fakis, J. S. Beckwith, K. Seintis, E. Martinou, C. Nançoz, N. Karakostas, I. Petsalakis, G. Pistolis, E. Vauthey Physical Chemistry Chemical Physics 20 (2018) 837-849
“Cooperative Self-Assembly Enables Two-Dimensional H-type Aggregation of a Sterically Crowded Perylene-Bisimide Dimer” N. Karakostas, V. S. Petrakis, F. Kournoutas, I. M. Mavridis, E. Martinou, E. Efthimiadou, M. Fakis, G. Pistolis, Cryst. Growth Des. 19 (2019) 4252-4263
Photophysics of star shaped and quadrupolar chromophores
Multibranched conjugated molecules are nowadays attractive materials for various optoelectronic applications such as organic light emitting diodes, solar energy conversion systems, sensors etc. due to their high energy harvesting ability, enhanced non-linear optical properties and high fluorescence quantum yield. A thorough investigation of their fundamental photophysical properties is of outmost importance and provides the necessary theoretical framework for designing novel light harvesting antennae materials with envisaged properties.
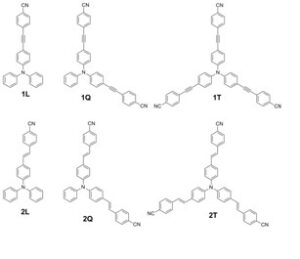 In a recent paper published in PCCP, we have studied the photophysical properties of two octupolar (T) molecules and their linear (L) and quadrupolar (Q) analogues by means of steady state and femtosecond to nanosecond spectroscopy. The compounds bear triphenylamine donor, cyano acceptors and acetylenic (series 1) or olefin (series 2) π-bridge. In the octupolar compound of series 2 (2T), fluorescence is emitted from an excited state localized on a single branch while in that of series 1 (1T), the emitting state is delocalized among
In a recent paper published in PCCP, we have studied the photophysical properties of two octupolar (T) molecules and their linear (L) and quadrupolar (Q) analogues by means of steady state and femtosecond to nanosecond spectroscopy. The compounds bear triphenylamine donor, cyano acceptors and acetylenic (series 1) or olefin (series 2) π-bridge. In the octupolar compound of series 2 (2T), fluorescence is emitted from an excited state localized on a single branch while in that of series 1 (1T), the emitting state is delocalized among 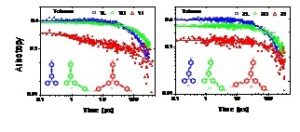 branches pointing to a reduced excited state polarity. Excited state dynamics in series 1 has shown an increase of lifetime with solvent polarity. In the branched compounds of series 2, multiexponential dynamics in polar solvents are exhibited indicating a distribution of emitting geometries. Femtosecond anisotropy in 1T indicates, an incoherent excitation transfer on few ps, in agreement with the hopping time predicted by the Förster model. However, no hopping mechanism is observed in 2T possibly because of an increased intramolecular charge transfer leading to a low energy relaxed excited state localized on a single branch.
branches pointing to a reduced excited state polarity. Excited state dynamics in series 1 has shown an increase of lifetime with solvent polarity. In the branched compounds of series 2, multiexponential dynamics in polar solvents are exhibited indicating a distribution of emitting geometries. Femtosecond anisotropy in 1T indicates, an incoherent excitation transfer on few ps, in agreement with the hopping time predicted by the Förster model. However, no hopping mechanism is observed in 2T possibly because of an increased intramolecular charge transfer leading to a low energy relaxed excited state localized on a single branch.
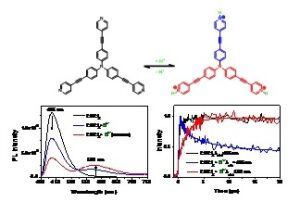 In another article published in J. Phys. Chem. A, a comparative study of the photophysical properties of octupolar pyridyl-terminated triphenylamine molecule, with its quadrupolar and dipolar analogues by means of ambient and low temperature steady state spectroscopy and fs to ns time resolved fluorescence spectroscopy was reported. The push-pull molecules bear triphenylamine electron donating core, pyridine peripheral electron acceptors and acetylenic π‑bridge. The samples were studied in solvents of varying polarity and also upon addition of small amounts of acetic acid to induce protonation of the pyridine group. All samples exhibit significant positive fluorescence solvatochromism as well as a relaxation of their excited state to a solvent relaxed Intramolecular Charge Transfer state on the ps timescale. For the octupolar compound, excited state relaxation occurs simultaneously with excitation energy hopping among the branches. The hopping time is solvent polarity controlled since it becomes slower as the polarity increases. The experimental hopping times are compared to those predicted by Förster and Fermi formulations. The samples are capable of emitting broadband light covering almost the whole visible spectrum by careful control of protonation. Energy transfer from the neutral towards the protonated species on the 1 ps timescale is revealed.
In another article published in J. Phys. Chem. A, a comparative study of the photophysical properties of octupolar pyridyl-terminated triphenylamine molecule, with its quadrupolar and dipolar analogues by means of ambient and low temperature steady state spectroscopy and fs to ns time resolved fluorescence spectroscopy was reported. The push-pull molecules bear triphenylamine electron donating core, pyridine peripheral electron acceptors and acetylenic π‑bridge. The samples were studied in solvents of varying polarity and also upon addition of small amounts of acetic acid to induce protonation of the pyridine group. All samples exhibit significant positive fluorescence solvatochromism as well as a relaxation of their excited state to a solvent relaxed Intramolecular Charge Transfer state on the ps timescale. For the octupolar compound, excited state relaxation occurs simultaneously with excitation energy hopping among the branches. The hopping time is solvent polarity controlled since it becomes slower as the polarity increases. The experimental hopping times are compared to those predicted by Förster and Fermi formulations. The samples are capable of emitting broadband light covering almost the whole visible spectrum by careful control of protonation. Energy transfer from the neutral towards the protonated species on the 1 ps timescale is revealed.
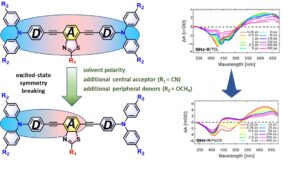
In the manuscript entitled “Exploring Solvent and Substituent Effects on the Excited State Dynamics and Symmetry Breaking of Quadrupolar Triarylamine End-Capped Benzothiazole Chromophores by Femtosecond Spectroscopy” published in JPCB we report on the investigation of the excited state dynamics and symmetry breaking process in three benzothiazole-derived two-photon absorbing chromophores by femtosecond fluorescence and transient absorption (fs-TA) spectroscopies in solvents of increasing polarity. Our measurements confirm that the extent of symmetry breaking is larger for D-π-A-π-D systems with the stronger acceptor core and increases further by increasing electron-donating strength of triarylamine moieties, giving rise to symmetry breaking of non-ionic quadrupolar molecules with ethynylene (triple bond) π-spacers also in less polar solvents.
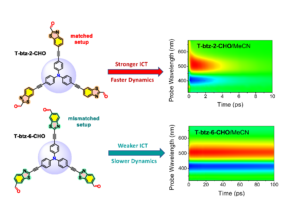
In the manuscript entitled “Ultrafast Photophysics of Regioisomeric Triphenylamine Dyes having Dipolar D-π-A-A and Octupolar D-(π-A-A)3 Character with a Benzothiazole Unit in Matched or Mismatched Orientation” we compare the photophysics and ultrafast dynamics of four octupolar molecules comprising a triphenylamine electron-donating core, ethynylene π-conjugated linkers and benzothiazole acceptors having the matched or mismatched orientation, while a carbaldehyde group is attached as an auxiliary acceptor. Among chromophores without the auxiliary acceptor, stronger fluorescence solvatochromism and faster excited state dynamics are exhibited for the derivatives with the mismatched geometry. On the contrary, introduction of the auxiliary acceptor to the benzothiazole unit enhances the Intramolecular Charge Transfer (featuring ultrafast dynamics of the excited state) for the matched geometry. The data confirm the crucial role of the relative orientation of asymmetric heteroaromatic unit in dipolar as well as in multipolar molecules in tuning linear and nonlinear optical properties as well as excited state dynamics.
see:
”Ultrafast Photophysics of Regioisomeric Triphenylamine Dyes having Dipolar D-π-A-A and Octupolar D-(π-A-A)3 Character with a Benzothiazole Unit in Matched or Mismatched Orientation”V. Petropoulos, I. Georgoulis, C. Vourdaki, P. Hrobárik, I. Sigmundová, J. Nociarová, M. Maiuri, G. Cerullo and M. Fakis 24, (2023) e202300127
“Exploring Solvent and Substituent Effects on the Excited State Dynamics and Symmetry Breaking of Quadrupolar Triarylamine End-Capped Benzothiazole Chromophores by Femtosecond Spectroscopy” M. Fakis, V. Petropoulos, P. Hrobárik, J. Nociarová, P. Osuský, M. Maiuri and G. Cerullo Journal of Physical Chemistry B 126, (2022), 8532–8543.
“Effect of protonation on the photophysical properties of 4-substituted and 4,7-disubstituted quinazoline push-pull chromophores” R. Plaza-Pedroche, D. Georgiou, M. Fakis, A. Fihey, C. Katan, F. Robin-le Guen, S. Achelle, J. Rodríguez-López, Dyes and Pigments 185 (2021) 108948
“Photophysics of 9,9-dimethylacridan substituted phenylstyrylpyrimidines exhibiting long lived intramolecular charge transfer fluorescence and aggregation induced emission characteristics” M. Fecková, I. K. Kalis, T. Roisnel, P. le Poul, O. Pytela, M. Klikar, F. Robin-le Guen, F. Bureš, M. Fakis, S Achelle, Chemistry A European Journal 13, (2021) 1145-1159
“The effect of protonation on the excited state dynamics of pyrimidine chromophores” F. Kournoutas, I. K. Kalis, M. Fecková, S. Achelle, M. Fakis, Journal of Photochemistry and Photobiology A: Chemistry 391 (2020) 112398
“Photophysical and Protonation Time Resolved Studies of DonorAcceptor Branched Systems With Pyridine Acceptors” F. Kournoutas, K. Seintis, N. Karakostas, J. Tydlitát, S. Achelle, G. Pistolis, F. Bureš, M. Fakis J. Physical Chemistry A 123 (2019) 417-428
“Femtosecond to nanosecond studies of octupolar molecules and their quadrupolar and dipolar analogues” K. Seintis, D. Agathangelou, D. Cvejn, N. Almonasy, F. Bureš, V. Giannetas and M. Fakis Physical Chemistry Chemical Physics 19 (2017) 16485-16497
Photophysics of quantum dots in solution and solid films
In recent works, we have investigated the optical properties of CuInS2/ZnS QDs. CuInS2 QDs are  non toxic and enable their application in Biology and Medicine. In solutions the QDs exhibit a red-shift of their absorption and photoluminescence spectra by increasing concentration and solvent polarity. In addition, they exhibit a three-exponential decay with time constants 1-3, 20-40 and 200-300 ns, depending on solvent, concentration and detection wavelength. In
non toxic and enable their application in Biology and Medicine. In solutions the QDs exhibit a red-shift of their absorption and photoluminescence spectra by increasing concentration and solvent polarity. In addition, they exhibit a three-exponential decay with time constants 1-3, 20-40 and 200-300 ns, depending on solvent, concentration and detection wavelength. In 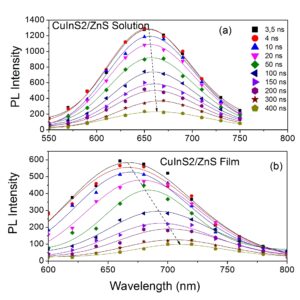 films, a red -shifted photoluminescence spectrum is observed for films made by drop-casting compared to those prepared by spin-coating. The timeresolved photoluminescence decays in films, apart from the three mechanisms observed in solutions, exhibit also a fast decay component of < 1 ns, which is more pronounced in the spin coated films and especially at long emission wavelengths. The presence of larger aggregates was found to lead to a larger PL red-shift. Besides, as the degree of aggregation increased, the PL decay became slower. We attribute the observed PL red-shift in solid fims to energy transfer from the smaller to the larger dots within the aggregates.
films, a red -shifted photoluminescence spectrum is observed for films made by drop-casting compared to those prepared by spin-coating. The timeresolved photoluminescence decays in films, apart from the three mechanisms observed in solutions, exhibit also a fast decay component of < 1 ns, which is more pronounced in the spin coated films and especially at long emission wavelengths. The presence of larger aggregates was found to lead to a larger PL red-shift. Besides, as the degree of aggregation increased, the PL decay became slower. We attribute the observed PL red-shift in solid fims to energy transfer from the smaller to the larger dots within the aggregates.
see:
“Energy transfer in aggregated CuInS2/ZnS core-shell quantum dots deposited as solid films” by S. Gardelis, M. Fakis, N. Droseros, D. Georgiadou, A. Travlos and A. Nassiopoulou Journal of Physics D: Applied Physics 50 (2017) 035107
“Steady state and time resolved photoluminescence properties of CuInS2/ZnS quantum dots in solutions and in solid films” N. Droseros, K. Seintis, M. Fakis, S. Gardelis and A. Nassiopoulou Journal of Luminescence 167 (2015) 333-338″